Danish physician Hans Christian Gram developed the Gram staining method in 1884. Gram staining procedure uses four chemicals; crystal violet, iodine, alcohol, and safranin, to stain bacteria
Gram staining is still the cornerstone of bacterial identification and taxonomic division. This differential staining technique separates most bacteria into two groups based on cell wall composition.
- Gram-positive bacteria- stains purple
- Gram-negative bacteria-stains red/pink
Nearly all clinically important bacteria can be visualized using the Gram staining technique, the only exceptions being those organisms;
- That exists almost exclusively within host cells, i.e., intracellular bacteria (e.g., Chlamydia)
- Those that lack a cell wall (e.g., Mycoplasma)
- Those of insufficient dimensions to be resolved by light microscopy (e.g., spirochetes)
Steps of Gram Staining
Classic Gram staining techniques involve the following steps:

- Fixation of clinical materials to the surface of the microscope slide either by heating or by using methanol. (Methanol fixation is recommended rather than heat fixation. Methanol fixation preserves the morphology of host cells and bacteria. Heating the slide causes cell distortion, could increase cell debris, and may cause erroneous Gram stain results.).
- Application of the primary stain (crystal violet). Crystal violet is a dark blue to purple dye. It stains all cells blue/purple.
- Application of mordant: The iodine solution (mordant) is added to form a crystal violet-iodine (CV-I) complex; all cells continue to appear blue.
- Decolorization step: The decolorization step distinguishes gram-positive from gram-negative cells. The organic solvent such as acetone or ethanol extracts the blue dye complex from the lipid-rich, thin-walled gram-negative bacteria to a greater degree than from the lipid-poor, thick-walled, gram-positive bacteria. The gram-negative bacteria appear colorless, and gram-positive bacteria remain blue.
- Application of counterstain (safranin): The red dye safranin stains the decolorized gram-negative cells red/pink; the gram-positive bacteria remain purple.
Find information and process for the Preparation of Gram Staining Regent
Principle of Gram Stain
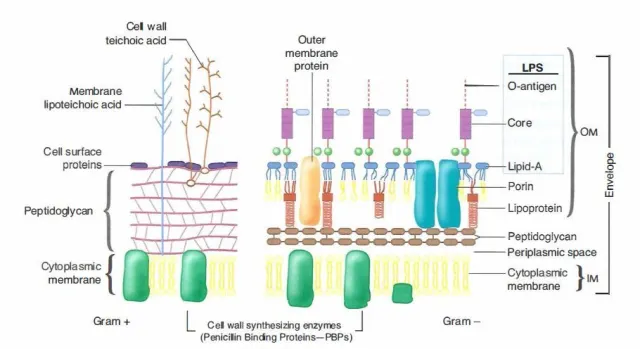
The differences in Gram-positive and Gram-negative bacteria cell wall composition account for the Gram staining differences. Gram-positive cell wall contains a thick layer of peptidoglycan with numerous teichoic acid cross-linking, which resists decolorization.
In aqueous solutions, crystal violet dissociates into CV+ and Cl – ions that penetrate through Gram-positive and Gram-negative cell walls. The CV+ interacts with negatively charged components of bacterial cells, staining the cells purple. When added, iodine (I- or I3-) interacts with CV+ to form large crystal violet-iodine (CV-I) complexes within the cytoplasm and outer layers of the cell.
The decolorizing agent (ethanol or an ethanol and acetone solution) interacts with the lipids of both gram-positive and gram-negative bacteria membranes.
- The outer membrane of the Gram-negative cell is lost from the cell, leaving the thin peptidoglycan layer exposed. With ethanol treatment, gram-negative cell walls become leaky and allow the large CV-I complexes to be washed from the cell.
- The highly cross-linked and multi-layered peptidoglycan of the gram-positive cell dehydrates after the addition of ethanol. Thus ethanol treatment traps the large CV-I complexes within the cell.
After decolorization, the **gram-positive cell remains purple. In contrast,**the gram-negative cell that has lost the purple color and is only visible when there is the addition of counterstain, the positively charged dye safranin. This weakly water soluble dye stain gram-negative bacteria as red. As safranin is lighter than crystal violet it doesn’t disrupt the purple coloration in Gram-positive cells.
Preparation of the smear
- Take a clean grease free slide.
- Transfer a loop of the sample (for example, sputum, CSF, or pus) to the microscope slide. If performing a Gram stain from a bacterial colony, first put a drop or a few loopful of water and emulsify the bacterial colony in the water drop.
- Spread the sample to an even-thin film over a circle of 15 mm diameter.
- Air dry the sample, and once the sample gets air dried, heat fix the smear by passing it through a bunsen burner three times. Heat application helps the cell adhesion (fixation) to the glass slide and prevents its loss during rinsing.
Allow the slide to cool to the touch before applying the stain. Alternatively, the smear can be fixed using methanol.
Methanol fixation
Place or hold the slide over a paper towel and flood the slide with absolute methanol for two minutes. Alternatively, dip the slide into a Coplin jar filled with methanol.
Once two minutes have passed, tilt the slide and drain off the excess methanol and let the slide air dry. Do not wipe or blot the slide, as this can remove cells.
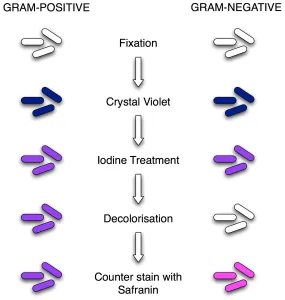
If you are struggling to remember the staining reagents used in this procedure and their order you can remember this sentence “ComeInAndStain” i.e. the order isCrystal violet,Iodine,Alcohol/Acetone and the final one isSafranin.
Gram Staining Procedure
The gram staining procedure involves four major steps; staining with crystal violet, fixing the dye, applying a decolorizer, and counter-staining.
- Flood air-dried, heat-fixed smear of cells for 1 minute with crystal violet staining reagent. Please note that the quality of the smear (too heavy or too light cell concentration) will affect the Gram Stain results.
- Wash slide in a gentle and indirect stream of tap water for 2 seconds.
- Flood slide with the mordant: Gram’s iodine. Wait 1 minute.
- Wash slide in a gentle and indirect stream of tap water for 2 seconds.
- Flood slide with decolorizing agent (acetone-alcohol decolorizer). Wait 10-15 seconds or add drop by drop to slide until the decolorizing agent running from the slide is clear.
- Flood slide with a counterstain, safranin. Wait 30 seconds to 1 minute.
- Wash slide in a gentile and indirect stream of tap water until no color appears in the effluent.
- **Allow the slide to air dry by tilting it onto a paper towel or over a sink.**Alternatively, gently dry the slide by blotting it using a lint-free bibulous paper.****Please do not use a wiping motion, as it can remove the smear.
- The slide is now ready to view under the microscope. First, focus on the image using the high dry objective lens marked 40x. Then, without removing the slide, switch to the high-power oil immersion objective lens marked 100x. Use immersion oil and observe the staining procedure’s resultsunder oil immersion (100x)using abright field microscope. This will result in an overall magnification of 1,000x.
After performing a gram stain, thetechnicianshould first determine whether the Gram stain is adequate. In an appropriately stained biological specimen, the nuclei of neutrophils are red. If the nuclei are blue, the decolorization is insufficient.
Results
- Gram-negative bacteria will stain pink/red and
- Gram-positive bacteria will stain blue/purple.
Reporting Gram smears
The report should include the following information:
- Numbers of bacteria present, whether many, moderate, few, or scanty
- Gram reaction of the bacteria, whether Gram-positive or Gram-negative
- Morphology of the bacteria, whether cocci, diplococci, streptococci, rods, or coccobacilli. Also, whether the organisms are intracellular.
- Presence and number of pus cells
- Presence of yeast cells and epithelial cells.
ExampleA urethral smear report might read: ‘Moderate numbers Gram-negative intracellular diplococci and many pus cells.’
Limitations
The sensitivity of the Gram stain procedure is low. Sometimes, you may fail to see the organism in Gram Stain smear, but the same clinical specimen may yield organisms when cultured. To be visible on a slide, organisms that stain by the Gram method must be present in about 104 to 105 organisms per milliliter of centrifuged fluid.
| Name | Reason | Alternative Microscopic Approach |
|---|---|---|
| Chlamydiae, including C. trachomatis | Intracellular; very small | Inclusion bodies in the cytoplasm |
| Legionella pneumophila | Poor uptake of red counterstain | Prolong time of counterstain |
| Mycoplasma pneumophila | Poor uptake of red counterstain | None |
| Mycobacterium, including M. tuberculosis | Too much lipid in cell wall so dye cannot penetrate | Acid-fast stain |
| Rickettsiae | Intracellular; very small | Giemsa or other tissue stains |
| Treponema pallidum | Too thin to see | Dark-field microscopy or fluorescent antibody |
Gram staining technique is not recommended for spirochetes and mycobacteria. Mycobacteria stain weakly with gram stain, and bacteria such as Mycoplasma, Rickettsiae, Chlamydiae do not take up the dyes used in Gram stain or are too small to be seen with light microscopy.
Not all bacteria can be visible in the Gram stain. This is the list of medically important bacteria that can be not been in Gram-stain.
Quality Control
Always check new batches of stain and reagents for correct staining reactions using a smear containing known Gram-positive and Gram-negative organisms.
Variations in Gram Reaction
Various factors influence the results of Gram staining. Sometimes the result might be entirely different than you have anticipated.
| Organism | Classic Presentation | Variant Presentation | Comments |
|---|---|---|---|
| Streptococccus pneumoniae | Gram-positive, lancet-shaped, diplococci | Elongated cocci, resembling short bacilli | May be misinterpreted as mixed organisms; over-decolorized cells may be mistaken for gram-negative coccobacilli. |
| Acinetobacter spp. | Gram-negative coccobacilli | Gram-negative cocci; gram-variable staining is common | May be mistaken for Neisseria spp. and reported as gram-negative cocci; search the smear to find some organisms that demonstrate elongated forms, which are not seen in Neisseria. |
| Clostridium perfringens | Boxcar-shaped gram-positive bacilli | Gram-positive cocci | May be mistaken for Streptococcus pneumoniae and reported as gram-positive cocci; in addition to a coccal form, cells retain crystal violet tenaciously during decolorization. |
| Clostridium perfringens | Boxcar-shaped gram-positive bacilli | Gram-variable or Gram-negative bacilli | Maybe mistaken for gram-negative bacilli; the boxcar shape is a clue that the organism is gram-positive; other Clostridia and Bacillus spp. May also appear similar. |
| Yeast, especially Cryptococcus neoformans | Gram-positive round or oval cells with budding | Gram-variable cells | May be mistaken for artifacts; size and shape distinguish them from bacteria. |
- Gram-positive bacteria may lose their ability to retain crystal violet and stain Gram negatively for the following reasons:
cell wall damage of bacteria due to antibiotic therapy or excessive heat fixation of the smear. over- decolorization of the smear use of an old Iodine solution that is yellow in color instead of brown (always store in a brown glass or other light opaque containers). preparation of smears from old culture.
- A thick smear will require more decoloration than a thin smear. When the smear is too thick, Gram-negative bacteria may not fully decolorize during decolorization steps and appear as Gram-positive.
Pitfalls in the Interpretation of Gram’s Stains
References and further reading
- Forbes, S., Sahm, D. F., & Weissfeld, A. S. (2002). Bailey & Scott’s Diagnostic Microbiology. Mosby.
- Levinson. (2010). Review of Medical Microbiology and Immunology. McGraw-Hill.
- Winn, W. C., & Koneman, E. W. (2006). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology (Color Atlas & Textbook of Diagnostic Microbiology). Lippincott Williams & Wilkins