Of all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable method. Moist heat has better penetrating power than dry heat and, at a given temperature, produces a faster reduction in the number of living organisms. Steam sterilization is nontoxic, inexpensive, rapidly microbicidal, and sporicidal. It rapidly heats and penetrates fabrics.
Sterilization is defined as killing or removal of all microorganisms including bacterial spores.
Moist heat sterilization using autoclave is commonly used for the sterilization of biohazardous trash, heat, and moisture resistant materials such as aqueous preparation (culture media). This method is also used for the sterilization of surgical dressings and medical devices.
The most common type of steam sterilizer in the microbiology laboratory is the gravity displacement type. Another type of autoclave is vacuum/gravity assisted.
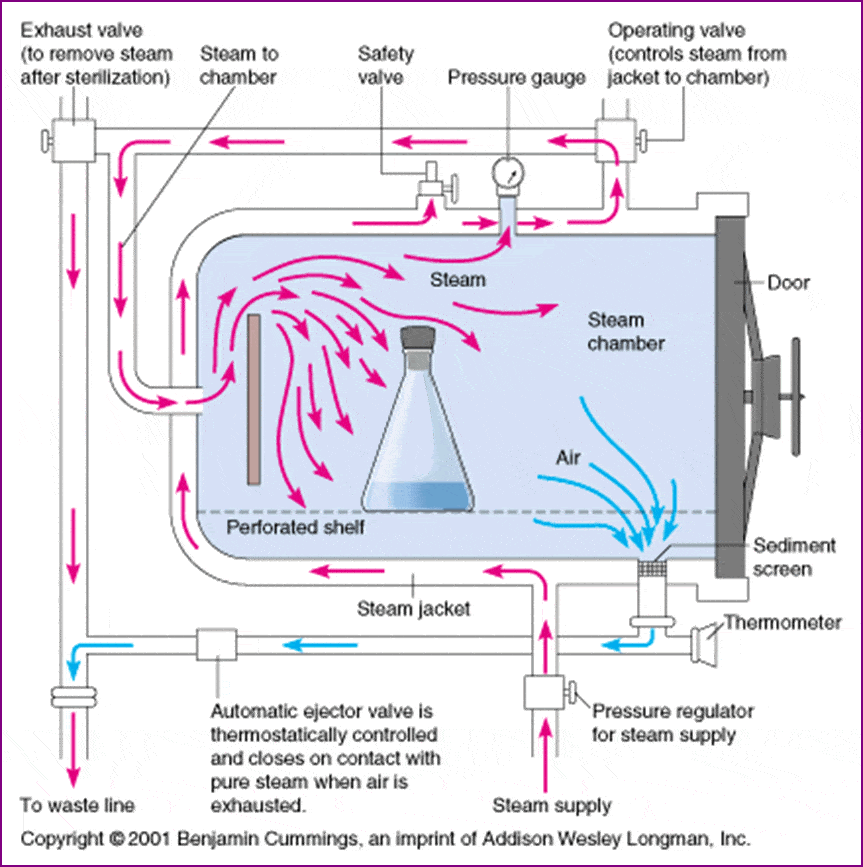
Principle of Moist Heat sterilization
Moist heat destroys microorganisms by the irreversible denaturation of enzymes and structural proteins. The temperature at which denaturation occurs varies inversely with the amount of water present. Sterilization in saturated steam thus requires precise control of time, temperature, and pressure.
| Temperature (°C) | Approximate corresponding pressure (kPa) | Minimum sterilization time (min) |
|---|---|---|
| 126-129 | 250 (~2.5 atm) | 10 |
| 134-138 | 300 (~3.0 atm) | 5 |
Pressure serves as a means to obtain the high temperatures necessary to quickly kill microorganisms. Specific temperatures must be obtained to ensure microbicidal activity. Minimum sterilization time should be measured from the moment when all the materials to be sterilized have reached the required temperature throughout.
The recommendation for sterilization in an autoclave is 15 minutes at 121°C (200 kPa). The temperature should be used to control and monitor the process; the pressure is mainly used to obtain the required steam temperature.
Alternative conditions, with different combinations of time and temperature, are given below.
1 1 atm = 325 Pa
In certain cases (e.g. thermolabile substances), sterilization may be carried out at temperatures below 121 °C, provided that the chosen combination of time and temperature has been validated.
Monitoring of steam sterilization process
Like other sterilization systems, the steam cycle is monitored by mechanical, chemical, and biological indicators. Steam sterilizers usually are monitored using a printout (or graphically) by measuring temperature, the time at the temperature, and pressure.
Chemical indicators are affixed to the outside and incorporated into the pack to monitor the temperature or time and temperature. Autoclave indicator tapes are commercially available and a change in color of the tape suggests proper sterilization.
Temperature-monitoring probes should be inserted into representative containers, with additional probes placed in the load at the potentially coolest and least accessible parts of the loaded chamber. The conditions should be within ±2 °C and ±10 kPa (±0.1 atm) of the required values. Each cycle should be recorded on a time-temperature chart or by other suitable means.
Biological Indicators
The effectiveness of steam sterilization is monitored with a biological indicator using an envelope containing spores of Geobacillus stearothermophilus (formerly Bacillus stearothermophilus; e.g. ATCC 7953 or CIP 52.81) for which the D-value (i.e. 90% reduction of the microbial population) is 1.5-2.5 minutes at 121 °C, using about 106 spores per indicator (this is based on a worst-case scenario that an item may contain a population of 106spores having same resistance as that of Bacillus stearothermophilus). After sterilization is over the strip is removed and inoculated into tryptone soy broth and incubated at 56°C for 5 days. No growth of Geobacillus stearothermophilus indicates proper sterilization.
| Spores of Bacteria | D Value |
|---|---|
| Geobacillus stearothermophilus (most common) | 1.5-2.5 |
| Bacillus coagulans | 0.3 |
| Clostridium sporogenes | 0.8-1.4 |
| Bacillus atropheus | 0.5 |
Table: list of commonly used biological indicators (BIs)
Positive spore test results are a relatively rare event and can be attributed to operator error, inadequate steam delivery, or equipment malfunction.
Advantages of Steam Sterilization Method
- Nontoxic to patient, staff, environment
- Cycle easy to control and monitor
- Rapidly microbicidal
- Least affected by organic/inorganic soils among sterilization processes listed
- Rapid cycle time
- Penetrates medical packing, device lumens
Disadvantages of Steam Sterilization Method
- Deleterious for heat-sensitive instruments
- Microsurgical instruments damaged by repeated exposure
- May leave instruments wet, causing them to rust
- Potential for burns
References and further readings