Diagnosis of visceral leishmaniasis may require taking a blood sample and/or taking a biopsy from the bone marrow or splenic puncture to show the parasite.
- Diagnosis of cutaneous leishmaniasis will require a small biopsy or scrapping of the ulcer.
- Diagnosis of mucocutaneous leishmaniasis requires a biopsy of the affected tissues.
Biopsy samples are examined by microscopy, culture, and other methods to look for the parasite and identify the specific kind of leishmania causing the ulcer.
Table of Contents
Sample for visceral leishmaniasis
- Blood: For making blood film/blood culture and serological tests.
- Bone Marrow: Biopsy material obtained by sternal or iliac crest puncture.
- Splenic Pulp: Biopsy material obtained by splenic culture.
As the clinical presentation of visceral leishmaniasis (kala-azar) lacks specificity, confirmatory tests are required to decide which patients should be treated. Such tests should be highly sensitive (>95%) as VL is a fatal condition, but also highly specific because the current drugs used to treat VL are toxic. Ideally, a test should be able to make the distinction between acute disease and asymptomatic infection, because none of the drugs currently available is safe enough to treat asymptomatic infections. Moreover, such tests should be simple and affordable.
Non-Leishmanial Test:
- Detection of reduction in the number of red and white blood cells and platelets (pancytopenia) in VL suspected clinical patients. This test is highly specific but sensitivity is very low.
- Aldehyde Test (also called Formal Gel Test: FGT): Polyclonal hypergammaglobulinemia (the production of high titers of non-specific antibodies) is detected in this test. The sensitivity of this test is poor, as low as 34%. In this test 1-2 drops of serum from a case of kala-azar are taken and a drop or two of formalin is added. A positive test is indicated by jellification to milk-white opacity like the white of a hard-boiled egg within 3 minutes to 24 hours.
Leishmanin Test
A killed culture (0.1-0.2 ml) of a suspension containing 6 to 10 promastigotes per ml is injected intradermally. A positive reaction (an area of induration after 72 hours) is produced in cured kala-azar cases 6 to 8 weeks after recovery and represents a delayed hypersensitivity reaction accompanied by cell-mediated immunity. The test is positive in African Kala-azar but not in Indian and Mediterranean kala-azar and post-kala-azar dermal leishmaniasis.
Parasite detection
For the detection of parasite visualization of the amastigote form of the parasite by microscopic examination of aspirates from lymph nodes, bone marrow, or spleen is done. It is the classic confirmatory test for visceral leishmaniasis.
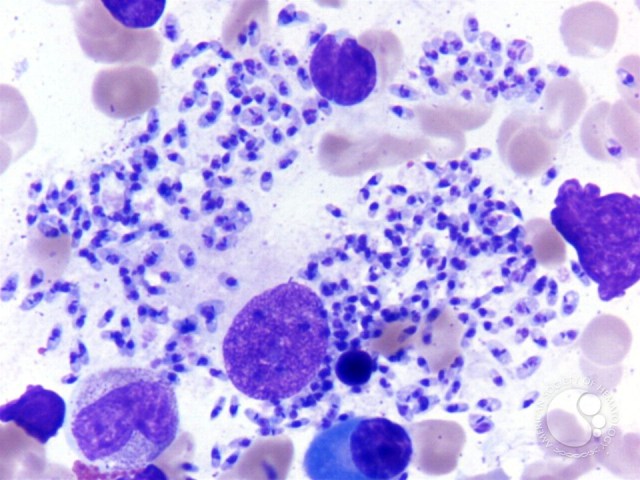
Although the specificity is high, the sensitivity of microscopy varies, being higher for the spleen (93% to 99%) than for bone marrow (53-86%) or lymph nodes aspirates (53-65%). The detection of parasites in the blood or organs by culture or by using molecular techniques such as PCR is more sensitive than microscopic examination.
A firm diagnosis of kala-azar/ visceral leishmaniasis (VL) requires demonstration of the parasite in splenic or bone marrow aspirate. But getting a biopsy sample is an invasive test so different antigen or antibody detection methods are in use for the diagnosis of visceral leishmaniasis.
Antibody detection tests
Antibody-based tests must be used in combination with a standardized clinical case definition for the diagnosis of visceral leishmaniasis. Serological tests based on indirect fluorescent antibody (IFA), enzyme-linked immunosorbent assay (ELISA), or western blot have shown high diagnostic accuracy in most studies but are poorly adapted for field settings. Two serological tests that have been specifically developed for field use and have been sufficiently validated are the direct agglutination test (DAT) and rK39-based immunochromatographic test (ICT).
Direct Agglutination Test (DAT)
Direct agglutination test (DAT) is another widely used test for serodiagnosis of kala-azar and is based on antigen-antibody reaction. Trypsin treated, stained, and formalin preserved promastigotes are used as antigens that show agglutination with specific antibodies present in patients’ serum. The test is performed at room temperature.
The usefulness of the above-mentioned serological test is limited by, its variable sensitivity or specificity, requirements of electricity, refrigeration, or a well-equipped laboratory, and high cost. Recently developed rapid dip-stick test – rK39 is the option available now to diagnose kala-azar cases at the grassroots in conjunction with the clinical diagnosis.
rK39 (Recombinant K39) Test
rk39 test (popularly known as K39 test) is a rapid, non-invasive test used for the diagnosis of visceral leishmaniasis (kala-azar). rK39 immunochromatographic tests are easily available in endemic countries.

K39 is an epitope (39 amino acid repeats encoded by a kinesin-like gene) apparently conserved on amastigotes of Leishmania species that cause visceral infection. By use of laboratory ELISA testing, circulating anti-K39, IgG is detectable in 95%- 100% of patients who have kala-azar, irrespective of geographic region.
Using K39 antigen-impregnated nitrocellulose strips developed for field conditions, fingerstick-obtained blood and serum samples demonstrated a sensitivity of 100% and a specificity of 97%. The strip testing proved simple to perform and yielded results within five minutes.

K39 strip test (ICT or dipstick format) is ideal for rapid reliable field diagnosis of visceral leishmaniasis. An rK39-based ELISA showed excellent sensitivity (93-100%) and specificity (97-98%) in many VL- endemic countries.
Antigen-detection tests
A heat-stable, low molecular weight carbohydrate antigen is detected by latex agglutination test in VL patients. It showed good specificity but only low to moderate (48-87%) sensitivity. Work to improve the format of this test is ongoing.
References and further readings
- Sastry A.S. & Bhat S. (2014) Essentials of Medical Parasitology. Jaypee Brothers Medical Publishers (P) Ltd
- Gracia, L.S. (2016). Diagnostic Medical Parasitology. ASM Press.



It is a very nice presentation . I like it very much . I am a medical technologist in lab. I am working in icddr,b . I want to know more about the in vitro culture of L.D bodies . Would you help me ? I am sending my email address to you.
I will be very glad to you .
Thanks again your nice presentation .