Infections of the upper respiratory tract usually involve the ears, the mucus membranes lining the nose and throat above the epiglottis, and the sinuses. Upper respiratory tract specimens should be collected within three days of symptom onset and no later than seven days, ideally before antimicrobial chemoprophylaxis or therapy.
Most infections involving the nose and throat are caused by viruses. Oropharyngeal (OP), nasopharyngeal (NP) swabs and nasopharyngeal wash/aspirate are the common upper respiratory tract specimens submitted for virologic assays. Nasopharyngeal wash/aspirate is the preferred sample.
Use a mask, gloves, and eye protection while collecting the specimen.
Materials
- Sterile Dacron/nylon swab with flexible plastic shaft. (Cotton-tipped or calcium alginate swabs are not acceptable)
- Universal Transport medium or Viral transport media (should contain 1-3 mL of sterile viral transport medium).
- Personal protection equipment, PPEs (i.e., mask, gloves, eye protection, gowns).
- Requisition form
- Biohazard label
- Biohazard bag
- Shipping container with cold packs
Note:Calcium alginate swab and swab with wooden shaft must not be used. These materials can inactivate viral particles and/or inhibit thePCR test. Furthermore, wooden shafts are likely to cause patient injury.
Procedure
Before Sample Collection
- Explain the procedure to the patient. Be sure to advise patients about potential discomfort during sample collection.
- Wash hands.
- Put on appropriate personal protective equipment (at a minimum, gloves and a facemask) to protect yourself in case the patient coughs or sneezes during sample collection. The level of PPEs depends on the infectiousness of the suspected pathogen. For example, in suspected coronavirus (SARS-CoV-2) infections, the examiner should wear an N-95 respirator mask, gown, protective goggles, and gloves.
- If the patient/resident has a lot of mucous in his/her nose, this can interfere with the collection of cells. Ask the patient to blow air into a tissue to clear excess secretions from the nasal passages.
- Seat the patient in a comfortable bed. It is best if the patient is placed in a high fowler’s position in bed with the back of the head supported. It may be necessary to have a second person available to assist with the collection.
Oropharyngeal (OP) Swab Collection
- Insert the swab into the posterior pharynx and tonsillar areas.
- Rub swab over both tonsillar pillars and posterior oropharynx and avoid touching the tongue, teeth, and gums.
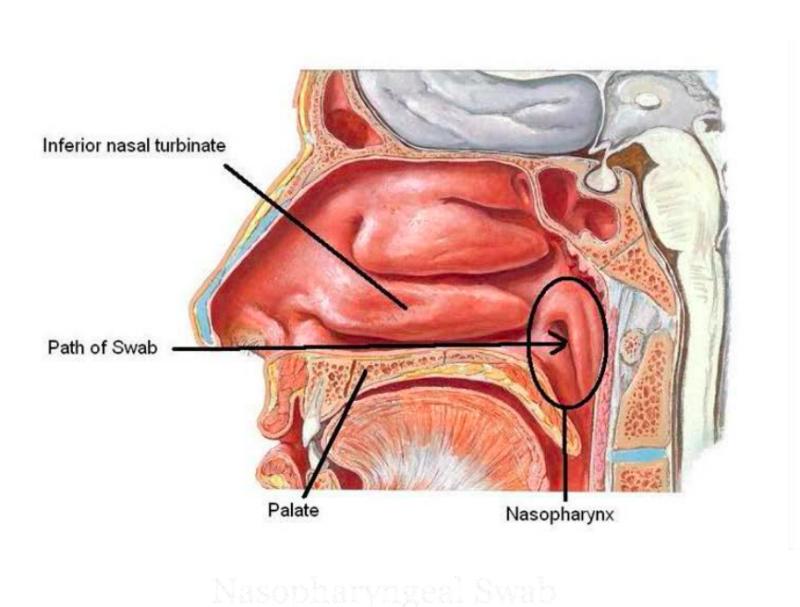
A Nasopharyngeal (NP) swab is the optimal upper respiratory tract specimen collection method for viral respiratory infections such as a respiratory syncytial virus (RSV), SARS-CoV-2, influenza virus A & B, and parainfluenza virus. Nasopharyngeal swabs are also collected for the diagnosis of bacterial infections such as Bordetella pertusis(whooping cough), Mycoplasma pneumoniaeand to screen carriers of meningococci.
Nasopharyngeal swab collection
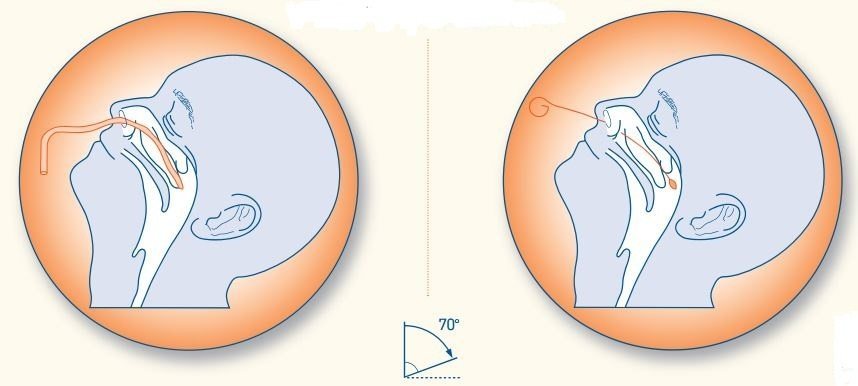
- Tilt the patient head back 70 degrees and support it with your non-dominant hand. If necessary lean the patient’s head against the wall to minimize jerky movements. Instruct the patient to close eyes to lessen the mild discomfort of the procedure.
- Stand slightly offset from the patient to avoid respiratory contamination in case of sudden cough or sneeze.
- Hold the swab like a pen between the thumb, index, and middle fingers.
- Start by inserting the swab horizontally into the left or right nostril. Carefully advance the swab while maintaining a course close to both the septum and floor of the nose, parallel to the palate, until the resistance (resistance is felt when the swab reaches the posterior nasopharynx) is felt. Ideally, you should collect two nasopharyngeal swabs. Note:The swab should reach adepth equal to the distance from the nostrils to the outer opening of the ear. Collecting samples from adult patients corresponds to a travel distance of about 5-6 cm or 2 inches.
- Leave the swab in place for several seconds to absorb secretions while gently wiping the wall by twisting the swab shaft for 10-15 seconds.
- Slowly and gently remove the swab.
- Immediately insert the swab into a sterile viral transport media tube, snap/cut off the applicator stick, replace the cap, and seal the tube tightly.
Place NP and OP swabs immediately into a sterile vial containing 2 ml of viral transport media. Both swabs can be placed in the same vial if desired. Aseptically, cut or break the applicator sticks off near the tip to permit the tightening of the cap.
Nasopharyngeal swab collection for laboratory diagnosis of COVID-19
SARS-CoV-2 is a highly infectious virus, so the above-mentioned nasopharyngeal swab collection procedure is modified, giving special care to the use of PPEs and extra steps to prevent the spread of the pathogen.
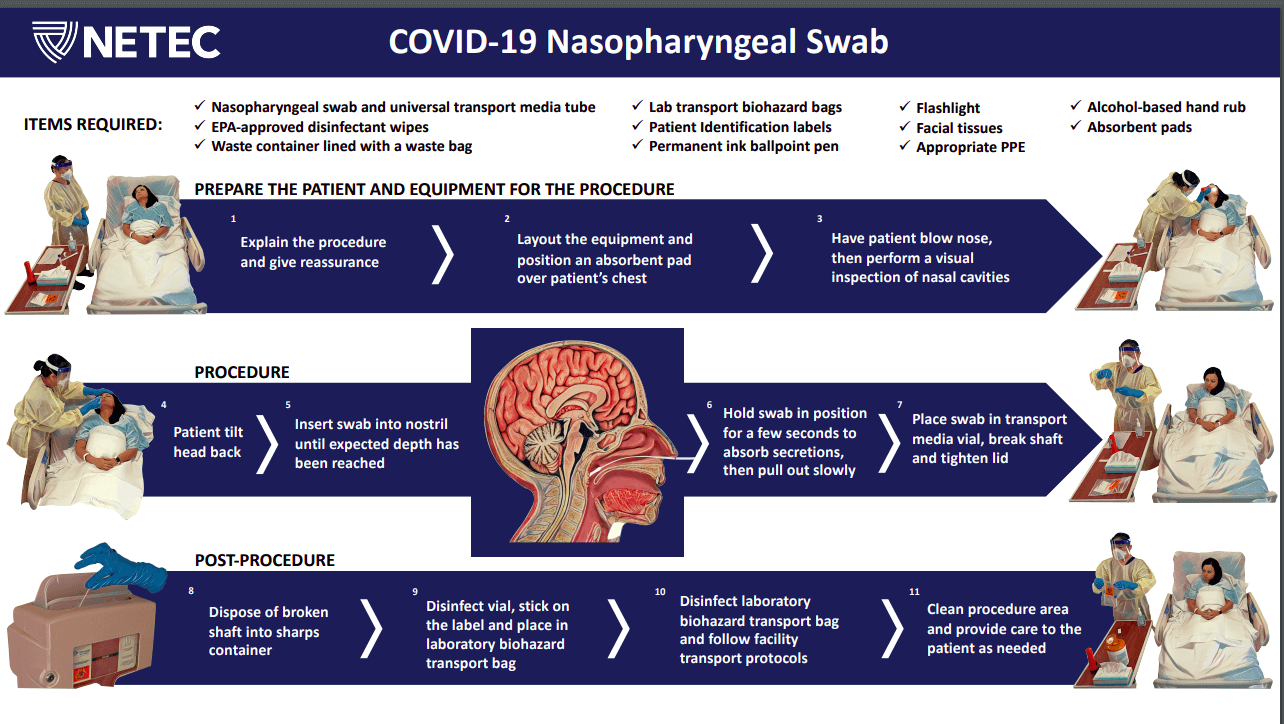
Nasopharyngeal wash/aspirateCollection
- Have the patient sit with the head tilted slightly backward.
- Instill 1 ml-1.5 ml of nonbacteriostatic saline (pH 7.0) into one nostril.
- Flush a plastic catheter or tubing with 2 ml-3 ml of saline.
- Insert the tubing into the nostril parallel to the palate (not upwards).
- Aspirate nasopharyngeal secretions. If permitted, repeat this procedure for the other nostril.
- Collect nasopharyngeal aspirate in sterile vials
Labeling
****Label each specimen container with:
- Name of the patient
- Hospital or lab identification number
- Type of specimen collected
- Date of collection
Transport and Storage
Send specimens to the lab immediately (testing sensitivity decreases over time). If samples will be examined within 48 hours after collection, keep specimen at 4oC and ship on wet ice or refrigerant gel-packs; otherwise, store frozen at ≤-70oC and ship on dry ice. The viability of some pathogens (e.g. respiratory syncytial virus) from specimens that are frozen and then thawed is greatly diminished and may result in false-negative test results.
Sample packaging and transport
- Label the specimen on viral transport media using a bar code or permanent marker.
- Place the specimen in a laboratory transport biohazard bag.
- Fill out the requisition form.
- Place the sample on refrigerator ice packs or at 4°C for transport and promptly transport to the laboratory. If delivery is delayed for more than four days, the specimen should be frozen at -70°C.
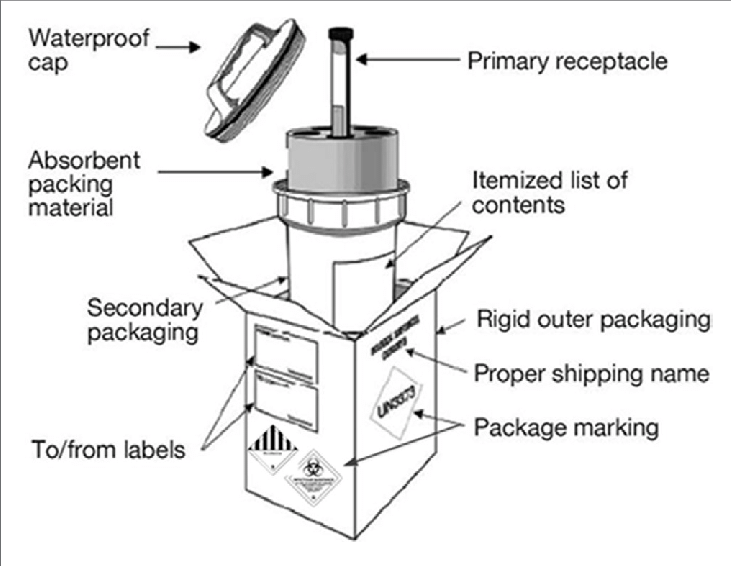
If there is a requirement for transportation or shipping of samples, samples should be packed in a basic triple packaging system with a primary watertight container wrapped with absorbent material, a secondary waterproof container, and an outer shipping package.
References and further readings
- CDC, “Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19),” NETEC Repository.
- World Health Organization. (2011). Manual for the laboratory diagnosis and virological surveillance of influenza. World Health Organization.
- Koneman’s Color Atlas and Textbook of Diagnostic Microbiology