Toxoplasma gondii is a protozoan parasite of many vertebrates including humans and causes the disease Toxoplasmosis. Its name was derived from the crescent shape of the tachyzoite stage of the parasite (taxon- ‘bow’; plasma- ‘form’). The parasite was first discovered in 1908, by Charles Nicolle and Louis Manceaux at the Pasteur Institute, in the North African rodent called the gundi, hence the species name gondii.
If a pregnant mother has a history of stillbirth or miscarriage she might be tested with ‘TORCH’ panel test. TORCH is an acronym representing congenital infections caused by Toxoplasma gondii, other agents, rubella, cytomegalovirus (CMV), and herpes simplex virus (HSV).
Table of Contents
Properties
- Obligate Intracellular parasite; reticuloendothelial cells.
- Worldwide in distribution; serologic evidence suggests more than 70% of all individuals are exposed to this pathogen but the disease itself is relatively rare.
- Most infections are benign and asymptomatic.
- Toxoplasma gondii completes its lifecycle into two hosts.
- Definitive hosts: members of family felidae (domestic cats and their relatives). The most common primary host of Toxoplasma is the cat.
- Intermediate hosts: Humans are the intermediate host. Other intermediate hosts are birds, rodents, pigs, cattle, etc.
Source of infection
Humans can become infected with Toxoplasma gondii via any of several routes:
- Ingestion of oocyst (containing sporozoites). This happens when a person consumes food or water contaminated with cat feces, for example while changing the litter box of a pet cat).
- Ingestion of undercooked meat of animals harboring tissue cysts (containing bradyzoites)
- Tachyzoites stage of this parasite can be transferred from infected person to susceptible person during blood transfusion or organ transplantation. Though rare, this is another possibility.
- Tachyzoites stage of this parasite can also transmit transplacentally from mother to fetus.
Morphological Forms
Toxoplasma gondii exists in three forms. All of these stages are infectious.
Oocyst
- Present in cat and other felines and not in humans.
- Formed by sexual reproduction.
- Oocyst shed in cat feces (contains two sporocyst, each containing 4 sporozoites).
(You know right? sporozoites are infective stages of malarial parasite).
Tachyzoites
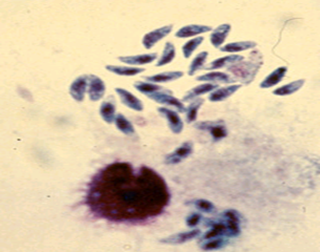
- Crescent-shaped with a pointed anterior and a rounded posterior end.
- Multiply rapidly in any cells of the intermediate hosts and non-enteric cells of definitive hosts.
- Active multiplying form seen during the acute stage of infection.
- Enters the host cell by active penetration of the host cell membrane.
- Group of proliferating tachyzoites within a host cell are known as pseudocyst.
Bradyzoites
- Occurs in chronic infection
- Asexual stages of the parasite
- Bradyzoites are found within the tissue cysts and multiply very slowly.
- Although the tissue cysts may develop in visceral organs such as the lungs, liver and kidneys, they are more prevalent in neural and muscular tissues, including the brain, eyes, and skeletal and cardiac muscles.
- Intact tissue cysts can persist for the life of the host and do not cause inflammatory response.
Life Cycle
An infected cat shed unsporulated oocysts in its feces for 1-2 weeks. After 1-5 days oocysts sporulate in the environment and become infective. Intermediate hosts in nature (including birds, rodents, and even humans) become infected after ingesting soil, water, or plant material contaminated with sporulated oocysts. Humans are also infected by the ingestion of raw meats, particularly pork, lamb, or venison.
Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in the neural and muscle tissue of intermediate hosts and develop into tissue cyst bradyzoites.
Cats become infected after
- consuming intermediate hosts (eg. rodents, birds, or raw meat) harboring tissue cysts (3-10 days developmental cycle).
- or directly by ingestion of sporulated oocysts (19-48 days developmental cycle).
In cats, some merozoites are transformed into the sexual stages, initiating gametogony. After sexual fusion of micro and macrogametes, oocysts develop, exit from the host cell into the gut lumen, and pass out via feces.
In the human host, the parasites form tissue cysts, most commonly in skeletal muscle, myocardium, brain, and eyes; these cysts may remain throughout the life of the host.
Signs and symptoms
Tachyzoites of the parasite Toxoplasma gondii may be found in circulating blood. They invade cells within lymph nodes and other organs, including the lungs, liver, heart, brain, and eyes. The resulting cellular destruction accounts for the manifestation of toxoplasmosis.
- Actively proliferating tachyzoites are seen in acute phase of infection and infect adjacent cells- continually expanding focal lesion.
- Formation of tissue cyst occurs as a result of host immune reaction. In this phase, there will be no multiplication, no dissemination of the parasite.
- Immunocompromised/immunodeficient individual: Cyst rupture or primary exposure to the organism may lead to the lesion. The organisms can be disseminated via the lymphatics and the bloodstream to other tissues.
Immunocompetent Patients
Usually asymptomatic or very mild infection in approximately 90% of serologically positive individuals.
Common symptoms
- No clinical symptoms in acte infections (in 80-90% of cases)
- Cervical lymphadenopathy (10-20% cases) and
- other symptoms of generalized infection (fever, malaise, night sweats, myalgia, sore throat and maculopapular rash).
Immunocompromised Patients
Infection in immunocompromised patients can lead to severe complications depending on the presence of underlying diseases such as malignancies, AIDS, and organ transplantation. In immunocompromised patients, the central nervous system (CNS) is primarily involved
Important symptoms
- Diffuse encephalopathy, meningoencephalitis, or cerebral mass lesions.
- >50% patients show an altered mental state, motor impariment, seizures, abnormal reflexes and other neurologic sequelae.
- These infections usually stem from reactivated latent infection, rather than newly acquired infection.
Congenital Infection
If a mother acquired infection before becoming pregnant unborn child is protected by the mother’s immunity. Mother’s blood contains IgG antibodies against T. gondii which can cross the placenta and reach the fetus.
If a mother is primarily infected with Toxoplasma during pregnancy or just before pregnancy, she can pass the infection on to the fetus. Congenital infections may be particularly severe if the mother acquires the infection during the first or second trimester of pregnancy. The mother may not have any symptoms from the infection or mild morbidity (flu-like illness) but the infant may develop serious symptoms later in life, such as blindness or mental disability.
Time determines the fate
- If Toxoplasma crosses the placenta early (1st trimester): severe congenital infections (intracerebral calcifications, chorioretinitis, hydro- or microcephaly, convulsions, mental retardation) may occur.
- If Toxoplasma crosses the placenta later (2nd-3rd trimester): infection may be inapparent but may lead to progressive blindness in the child later in life (teens).
- Secondary Infection: Maternal antibodies (secondary infection) protect the fetus during pregnancy, even if the mother is re-exposed during pregnancy.
Laboratory Diagnosis
Diagnosis of toxoplasmosis is usually achieved by serology. Test for the presence of specific IgG or IgM is used to determine if a person has an acute infection with Toxoplasma or immunity due to prior infection. Other procedures include performing PCR, examining biopsy specimens, buffy coat cells or CSF fluid, and isolating the organism in tissue culture or in laboratory animals.
Laboratory diagnosis methods
- Seroloy
- Giemsa stain and examining biopsy specimens, buffy coat cells, or cerebrospinal fluid
- Isolating the organism in tissue culture or in laboratory animals.
- Molecular methods: Performing PCR
Serology
- Serological tests are often recommended as diagnostic approach of choice but the interepretation of serological tests are complex.
- Specific IgM normally develops early, within 1-2 weeks after primary infection and IgG normally develops within 4 weeks after infection. Antibodies titer may peak within 4-8 weeks of infection.
- A four fold rise in IgG antibody titer is required to support the diagnosis of acute febrile toxoplasmosis.
- All newborns from mother who are antibody positive will have passively transferred maternal IgG. Titers can be high but may not indicate infection in in the baby.
- Detection of IgM antibodies (which do not cross the placenta) provides a much more accurate indication of infection in the newborn.

(Chart source: European Journal of Obstetrics and Gynecology and Reproductive Biology)
Sabin-Feldman dye test
- One of the first methods used to diagnose toxoplasmosis
- In the presence of immune serum, T. gondii loses its affinity for methylene blue stain.
- Not done routinely in diagnostic laboratories
- uses live Toxoplasma trophozoites.
Microscopy
- Smears or sections stained with Giemsa or other special stains eg. PAS.
- Tachyzoites of T. gondii in the smear are cresent shaped (malarial parasite Plasmodium falciparum also has crescent shaped gaemtocytes) and in sections round to oval
- Tissue cysts are usually spherical and lack septa
Culture
T. gondii can be isolated by intraperitoneal inoculation of body fluid or tissue in infection-free laboratory mice. Peritoneal fluid and spleen smears may show the tachyzoites after 7-10 days.
Molecular methods
Detection of a specific region of DNA of Toxoplasma in the sample (eg. Blood, CSF, etc). Prenatal diagnosis of congenital toxoplasmosis from amniotic fluid.
References and further readings
- Gracia, L.S. (2016). Diagnostic Medical Parasitology. ASM Press.
- Toxoplasmosis (Toxoplasma infection). Centers for Disease Control and Prevention.