Kato Katz technique is used for qualitative and semi-quantitative diagnosis of intestinal helminthic infestations; caused by Ascaris lumbricoides, Trichuris trichiura, hookworm, and especially Schistosoma spp. WHO has recommended the Kato Katz technique in areas with moderate to high transmission rates of soil-transmitted helminths (i.e. where the proportion of infected individuals is >20– >50%) or intestinal schistosomiasis (>10–50%). Where the prevalence of soil-transmitted helminths (STH) is <20%, the specificity of this technique makes it less appropriate, and more sensitive tools should be used.
Direct wet mount is the commonly used method for the diagnosis of intestinal parasitic infections but its sensitivity is low. Formal ether concentration (FEC) technique is used to increase the sensitivity of the microscopic detection methods.
Table of Contents
Principle
People infected with STH or intestinal schistosomes pass the eggs of the worms through their feces. In the Kato-Katz technique, feces are pressed through a mesh screen to remove large particles. A portion of the sieved sample is then transferred to the hole of a template on a slide. After filling the hole, the template is removed and the remaining sample is covered with a piece of cellophane soaked in glycerol. The glycerol clears the fecal material from around the eggs. The eggs are then counted and the number is calculated per gram of feces.
Materials
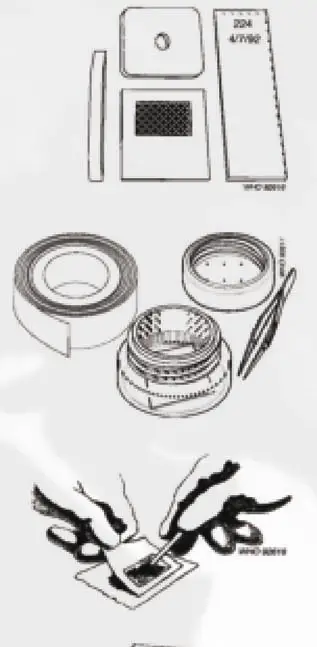
- Kato-set (template with hole, screen, nylon or plastic, plastic spatula)
- Newspaper or glazed tile
- Microscope slides
- Cellophane as coverslip, soaked in a glycerol-malachite green or glycerol-methylene blue solution.
- Fresh stool
- Gloves
Procedure of Kato-Katz Technique
- Label a glass slide with the sample number and then place a plastic template on top of it.
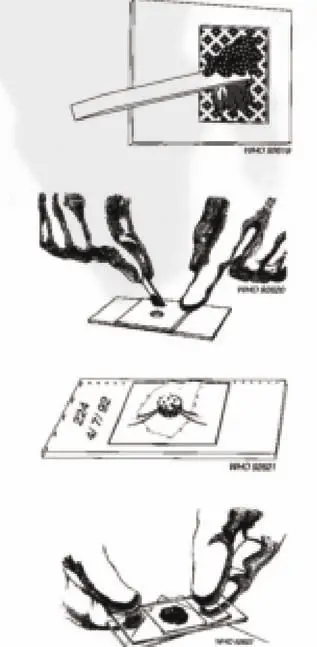
- Place a small amount of the fecal sample on a newspaper and press a piece of nylon screen on top. Using a spatula, scrape the sieved fecal material through the screen so that only the debris remains.
- Scrape up some of the sieved feces to fill the hole in the template, avoiding air bubbles and leveling the feces off to remove any excess.
- Carefully lift off the template and place it in a bucket of water mixed with concentrated detergent so that it can be reused.
- Place one piece of the cellophane, which has been soaked overnight in methylene blue glycerol solution, over the fecal sample.
- Place a clean slide over the top and press it evenly downwards to spread the feces in a circle. Carefully remove the slide by gently sliding it sideways to avoid separating the cellophane strip. If done well, it should be possible to read newspaper print through the stool smear. Place the slide with the cellophane upwards.
Note: If hookworm is present in the area the slide should be read within 30–60 minutes. After that time, the hookworm eggs disappear.
Examination and Results
- Place the slide under a microscope and examine the whole area in a systematic zigzag pattern.
- Record the number and the type of each egg of each species on a recording form alongside the sample number.
- Finally, multiply the number of eggs by the appropriate number (see inlet-information of the kato-set) to give the number of eggs per gram (epg) – the standard measurement to assess the intensity of infection.
Further reading and reference