Bacitracin is a bactericidal drug that is useful in treating superficial skin infections but is too toxic for systemic use. Bacitracin is a polypeptide antibiotic produced by Bacillus subtilis.
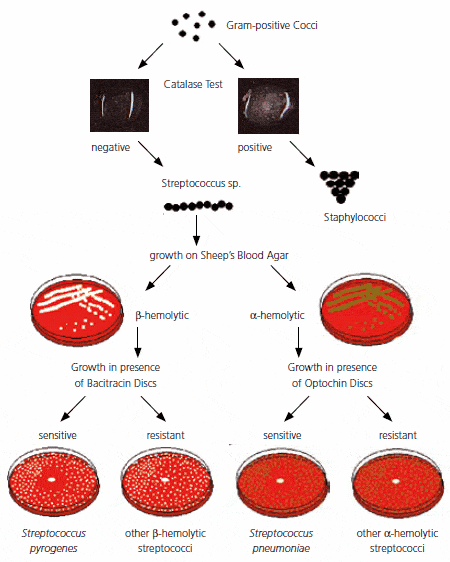
Bacitracin interferes with the peptidoglycan synthesis of bacteria. The presumptive identification of group A streptococci (GAS) is usually made by testing for sensitivity to bacitracin.
Principle
Bacitracin test is used to determine the effect of a small amount of bacitracin (0.04 IU or 0.05 IU not higher) on an organism. Streptococcus pyogenes (Group A Streptococci)is inhibited by the small amount of bacitracin in the disk; other beta-hemolytic streptococci usually are not. Some laboratories do not recommend the use of 0.04 U bacitracin disk as Lancefield groups C and G streptococci may occasionally also show susceptibility to bacitracin. PYR reaction can confirm the isolate as S. pyogenesas it’s the only beta-hemolytic streptococci that gives a positive PYR reaction.
Quality Control
Perform sterility and performance testing blood agar plate and/or chocolate agar plate according to CLSI guidelines. Test the disk potency after each shipment or purchase of the bacitracin disk with the appropriate test organism.
| Test organism | Bacitracin (10 IU) zone size |
|---|---|
| Haemophilus influenzae ATCC 10211 | No zone |
| Staphylococcus epidermidis ATCC 12228 | 12 mm |
Procedure of Bacitracin test
- Using an inoculating loop, streak two or three suspect colonies of a pure culture onto a blood agarplate.*****
- Using heated forceps, place a bacitracin disk in the first quadrant (area of heaviest growth). Gently tap the disk to ensure adequate contact with the agar surface.
- Incubate the plate for 18 to 24 hours at 35°C in CO2.
- Look for a zone of inhibition around the disk.
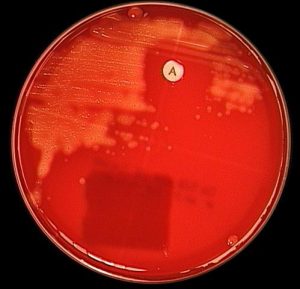
*****Note:If used on direct sputum culture plates, usechocolate agarfor bacitracin and blood agar plate foroptochin. Addition of bacitracin disk (not Taxo A) to chocolate agar inhibits upper respiratory microbiota and improves detection of Haemophilus influenzae.
Results
- Bacitracin sensitive: Any zone of inhibition around the disk. For example, Streptococcus pyogenes
- Bacitracin resistant: No zone of inhibition around the disk. Streptococcus agalactiae
Image sources:
- Image 1: sigmaaldrich.com
- Image 2: University of Florida
References
- Murray, P. R., Wold, A. D., Hall, M. M., & Washington, J. A., 2nd (1976). Bacitracin differentiation for presumptive identification of group A beta-hemolytic streptococci: comparison of primary and purified plate testing. The Journal of pediatrics, 89(4), 576–579. https://doi.org/10.1016/s0022-3476(76)80389-7
- Katz, B. E., & Fisher, A. A. (1987). Bacitracin: a unique topical antibiotic sensitizer. Journal of the American Academy of Dermatology, 17(6), 1016–1024. https://doi.org/10.1016/s0190-9622(87)70292-8