Hematopoiesis, derived from the Greek words haima (blood) and poiēsis (to produce), is the process of forming blood cells, including red blood cells (RBCs), white blood cells (WBCs), and platelets. This vital process begins in the embryonic stage and continues throughout life, ensuring a constant supply of blood cells to support oxygen transport, immune function, and clotting. For microbiology students, understanding hematopoiesis is key to grasping how the body maintains its blood cell populations and responds to infections or injuries.
Stages of Hematopoiesis
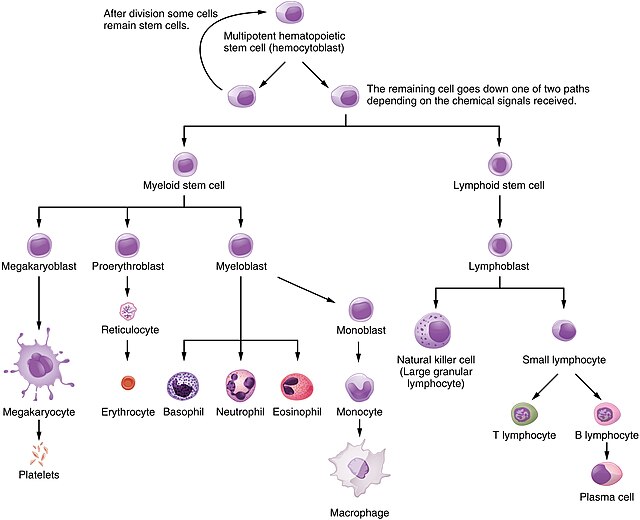
Hematopoiesis is a dynamic process that occurs in different locations and stages throughout life, driven by hematopoietic stem cells (HSCs). These multipotent cells can self-renew or differentiate into progenitor cells, which mature into specialized blood cells.
Embryonic Hematopoiesis
- Primitive Hematopoiesis: Occurs in the yolk sac starting around day 19 of conception in humans, producing primitive erythrocytes for oxygen transport and myeloid cells (e.g., microglia, Langerhans cells) for early immune function. This phase lasts until approximately the 8th week.
- Intraembryonic Hematopoiesis: Takes place in the aorta-gonad-mesonephros (AGM) region, where HSCs arise from the ventral endothelial wall of the dorsal aorta. These cells later express markers like CD45 and MHC II in adulthood.
- Fetal Hematopoiesis: Around 5–8 weeks of gestation, hematopoiesis shifts to the liver and spleen, where HSCs differentiate into myeloid and lymphoid lineages. By 16–20 weeks, bone marrow becomes the primary site (medullary hematopoiesis), continuing into adulthood.
Adult Hematopoiesis
In adults, hematopoiesis primarily occurs in the bone marrow of the skull, pelvic bones, vertebrae, and long bone metaphyses. In children, active bone marrow is more extensive due to higher RBC demand. In pathological conditions (e.g., bone marrow failure), hematopoiesis may revert to embryonic sites like the liver or spleen.
Hematopoietic Stem Cells (HSCs)
HSCs are specialized, multipotent cells in the bone marrow and peripheral blood capable of:
- Self-Renewal: Producing identical HSCs to maintain their population.
- Differentiation: Developing into progenitor cells that form mature RBCs, WBCs, or platelets.
HSCs can remain quiescent (dormant) to avoid exhaustion, activating in response to injury or infection to increase blood cell production. Two models explain their fate:
- Stochastic Model: HSC differentiation is random.
- Instructive Model: Microenvironmental signals (e.g., cytokines) guide differentiation.
HSCs include:
- Long-Term HSCs: Primarily self-renew, ensuring a lifelong stem cell pool.
- Short-Term HSCs: Differentiate into multilineage progenitors, such as common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs).
Hematopoietic Microenvironment (Niche)
The bone marrow microenvironment, or niche, regulates hematopoiesis through cellular and molecular interactions. Key components include:
Bone Marrow Stromal Cells
- Fibroblasts, Adipocytes, Endothelial Cells, Osteoblasts: Provide structural support and secrete signaling molecules.
- Function: Support HSC survival, proliferation, and differentiation.
Cytokines and Growth Factors
- Stem Cell Factor (SCF): Promotes HSC survival via c-Kit receptor.
- Interleukins (IL-3, IL-6): Support progenitor cell proliferation.
- Granulocyte Colony-Stimulating Factor (G-CSF): Stimulates granulocyte production.
- Erythropoietin (EPO): Drives RBC production in response to low oxygen levels.
- Thrombopoietin (TPO): Regulates platelet production.
Extracellular Matrix (ECM)
- Composed of proteins like collagen, fibronectin, and laminin.
- Binds to HSC integrins, regulating cell adhesion, migration, and behavior.
Niches
- Endosteal Niche: Rich in osteoblasts, maintains HSC quiescence.
- Vascular Niche: Contains endothelial and perivascular cells, promotes HSC activation and mobilization.
Hematopoiesis Processes
HSCs differentiate into two main lineages:
- Common Myeloid Progenitors (CMPs): Produce RBCs (erythropoiesis), platelets (thrombopoiesis), and myeloid cells like granulocytes (neutrophils, basophils, eosinophils) or agranulocytes (macrophages, monocytes) via myelopoiesis.
- Common Lymphoid Progenitors (CLPs): Form lymphocytes (T, B, NK cells) through lymphopoiesis.
Each process is identified by specific cell surface markers (e.g., CD markers) for stem cells, progenitors, and mature cells.
Regulation of Hematopoiesis
Hematopoiesis is tightly regulated by intrinsic and extrinsic factors to maintain balanced blood cell production.
Intrinsic Regulators
- Transcription Factors:
GATA-1: Drives erythroid and megakaryocyte differentiation. PU.1: Supports myeloid and lymphoid development. RUNX1: Regulates HSC development and all lineages.
- Epigenetic Modifications:
DNA Methylation: Controls gene expression for lineage commitment. Histone Modifications: Affect chromatin structure and gene activity. MicroRNAs: Regulate HSC maintenance and differentiation.
- Signaling Pathways:
Notch Signaling: Influences HSC fate via cell-cell interactions. Wnt Signaling: Regulates self-renewal and differentiation.
Extrinsic Regulators
- Cytokines/Growth Factors: SCF, EPO, G-CSF, and TPO drive specific cell production.
- Cell-Cell Interactions: Stromal cells, osteoblasts, and endothelial cells interact with HSCs via adhesion molecules (e.g., integrins, cadherins).
- Systemic Factors:
Hormones: Glucocorticoids and thyroid hormones influence hematopoiesis. Nutritional Status: Vitamins (B12, folate) and iron are essential. Immune Signals: Cytokines (e.g., interleukins) boost immune cell production during infection.
Feedback Mechanisms
- Negative Feedback: High RBC levels reduce EPO production; mature immune cells inhibit further production via cytokines.
- Homeostatic Balance: Ensures physiological blood cell levels.
Clinical Relevance of Hematopoiesis
Disruptions in hematopoiesis can lead to serious disorders, impacting blood cell production and function.
Anemias
- Aplastic Anemia: Bone marrow failure causing pancytopenia (low RBCs, WBCs, platelets). Symptoms: fatigue, infections, bleeding. Treatments: immunosuppressive therapy, bone marrow transplants.
- Iron-Deficiency Anemia: Reduced hemoglobin due to low iron. Symptoms: fatigue, pallor. Treatments: iron supplements, dietary changes.
- Megaloblastic Anemia: Vitamin B12/folate deficiency causing abnormal RBCs. Symptoms: fatigue, neurological issues. Treatments: B12/folate supplements.
Leukemias
- Acute Myeloid Leukemia (AML): Excessive myeloid blasts. Symptoms: fatigue, infections. Treatments: chemotherapy, bone marrow transplants.
- Acute Lymphoblastic Leukemia (ALL): Lymphoid precursor proliferation. Symptoms: fever, bone pain. Treatments: chemotherapy, targeted therapies.
- Chronic Myeloid Leukemia (CML): Caused by BCR-ABL gene. Symptoms: fatigue, splenomegaly. Treatments: tyrosine kinase inhibitors (e.g., imatinib).
- Chronic Lymphocytic Leukemia (CLL): B lymphocyte proliferation. Symptoms: lymphadenopathy, fatigue. Treatments: chemotherapy, targeted therapies.
Myelodysplastic Syndromes (MDS)
Ineffective hematopoiesis with AML risk. Symptoms: anemia, infections. Treatments: transfusions, growth factors, stem cell transplants.
Myeloproliferative Neoplasms (MPNs)
- Polycythemia Vera (PV): Excessive RBCs due to JAK2 mutation. Symptoms: headaches, thrombosis risk. Treatments: phlebotomy, JAK2 inhibitors.
- Essential Thrombocythemia (ET): Platelet overproduction. Symptoms: thrombosis, bleeding. Treatments: aspirin, cytoreductive therapy.
- Primary Myelofibrosis (PMF): Bone marrow fibrosis. Symptoms: anemia, splenomegaly. Treatments: JAK2 inhibitors, stem cell transplants.
Lymphomas
- Hodgkin Lymphoma: B cell malignancy with Reed-Sternberg cells. Symptoms: lymphadenopathy, fever. Treatments: chemotherapy, radiation.
- Non-Hodgkin Lymphoma: Diverse lymphoid malignancies. Symptoms: lymphadenopathy, weight loss. Treatments: chemotherapy, targeted therapies.
Bone Marrow Failure Syndromes
- Fanconi Anemia: Genetic disorder causing pancytopenia. Symptoms: congenital anomalies, cancer risk. Treatments: stem cell transplants.
- Diamond-Blackfan Anemia: Congenital RBC deficiency. Symptoms: severe anemia. Treatments: corticosteroids, transfusions.
Hematopoiesis in Clinical Practice
- Leukemia: Malignant cells disrupt the bone marrow niche, impairing normal hematopoiesis.
- Bone Marrow Transplantation: Requires a supportive niche for donor HSCs, using conditioning regimens and growth factors for engraftment.
References
- Jagannathan-Bogdan, M., & Zon, L. I. (2013). Hematopoiesis. Development, 140(12), 2463–2467. https://doi.org/10.1242/dev.083147
- Chapman, J., & Zhang, Y. (2023). Histology, Hematopoiesis. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK534246/
- Morrison, S. J., Uchida, N., & Weissman, I. L. (1995). The biology of hematopoietic stem cells. Annual Review of Cell and Developmental Biology, 11, 35–71. https://doi.org/10.1146/annurev.cb.11.110195.000343
- Greenberger, J. S. (1991). The hematopoietic microenvironment. Critical Reviews in Oncology/Hematology, 11(1), 65–84. https://doi.org/10.1016/1040-8428(91)90018-8
- Gaballa, M., & Ramos, C. A. (2019). Overview of normal hematopoiesis. Handbook of Benign Hematology. https://doi.org/10.1891/9780826149879.0001